Assemblies
Assembly Name:
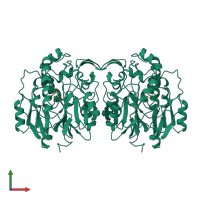
Glycine amidinotransferase, mitochondrial
Multimeric state:
homo dimer
Accessible surface area:
23886.44 Å2
Buried surface area:
3467.14 Å2
Dissociation area:
1,473.39
Å2
Dissociation energy (ΔGdiss):
4.22
kcal/mol
Dissociation entropy (TΔSdiss):
13.93
kcal/mol
Symmetry number:
2
PDBe Complex ID:
PDB-CPX-156180
Macromolecules
Chain: A
Length: 423 amino acids
Theoretical weight: 48.52 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
InterPro: Glycine/inosamine-phosphate amidinotransferase
CATH: L-arginine/glycine Amidinotransferase; Chain A
SCOP: Amidinotransferase
Length: 423 amino acids
Theoretical weight: 48.52 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
- Canonical:
 P50440 (Residues: 1-423; Coverage: 100%)
P50440 (Residues: 1-423; Coverage: 100%)
InterPro: Glycine/inosamine-phosphate amidinotransferase
CATH: L-arginine/glycine Amidinotransferase; Chain A
SCOP: Amidinotransferase