Function and Biology Details
Reactions catalysed:
Arachidonate + O(2) = (5Z,8Z,10E,14Z)-(12S)-12-hydroperoxyicosa-5,8,10,14-tetraenoate
Arachidonate + O(2) = (5Z,8Z,11Z,13E)-(15S)-15-hydroperoxyicosa-5,8,11,13-tetraenoate
Biochemical function:
Biological process:
Cellular component:
- not assigned
Structure analysis Details
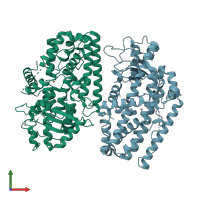
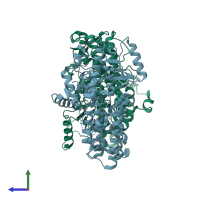
Assembly composition:
monomeric (preferred)
Assembly name:
Polyunsaturated fatty acid lipoxygenase ALOX12 (preferred)
PDBe Complex ID:
PDB-CPX-148273 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule: