X-ray diffraction
1.43Å resolution
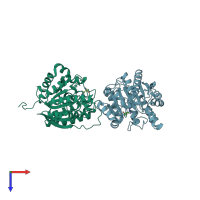
Crystal of human arginase in complex with L-ornithine. Resolution 1.43 A.
Released:
DOI:
10.2210/pdb3gmz/pdb
Source organism:
Homo sapiens
Primary publication: