Function and Biology Details
Reactions catalysed:
Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1)
The C-O-P bond 3' to the apurinic or apyrimidinic site in DNA is broken by a beta-elimination reaction, leaving a 3'-terminal unsaturated sugar and a product with a terminal 5'-phosphate
Biochemical function:
Biological process:
Cellular component:
Sequence domains:
Structure analysis Details
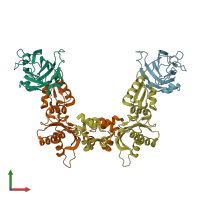
Assembly composition:
hetero dimer (preferred)
Assembly name:
DNA repair protein XRCC1 and DNA polymerase beta (preferred)
PDBe Complex ID:
PDB-CPX-139218 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):
Ligands and Environments
No bound ligands
No modified residues
Experiments and Validation Details
X-ray source:
RIGAKU MICROMAX-007 HF
Spacegroup:
C2
Expression system: Escherichia coli