Assemblies
Assembly Name:
67 kDa matrix metalloproteinase-9
Multimeric state:
homo dimer
Accessible surface area:
16926.8 Å2
Buried surface area:
4601.79 Å2
Dissociation area:
735.36
Å2
Dissociation energy (ΔGdiss):
9.1
kcal/mol
Dissociation entropy (TΔSdiss):
12.13
kcal/mol
Symmetry number:
2
PDBe Complex ID:
PDB-CPX-147089
Macromolecules
Chains: A, B
Length: 160 amino acids
Theoretical weight: 17.93 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
Pfam: Matrixin
InterPro:
Length: 160 amino acids
Theoretical weight: 17.93 KDa
Source organism: Homo sapiens
Expression system: Escherichia coli
UniProt:
- Canonical:
 P14780 (Residues: 110-444; Coverage: 23%)
P14780 (Residues: 110-444; Coverage: 23%)
Pfam: Matrixin
InterPro:
- Metallopeptidase, catalytic domain superfamily
- Peptidase, metallopeptidase
- Peptidase M10, metallopeptidase
- Peptidase M10A, catalytic domain
- Peptidase M10A



![The deposited structure of PDB entry <span class='highlight'>4hma</span> coloured by chain, front view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>67 kDa matrix metalloproteinase-9</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>CALCIUM ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>N,N'-bis(2-[(biphenyl-4ylsulfonyl)[(2R)-1-hydroxy-3-methyl-1-oxobutan-2-yl]-amino]ethyl)benzene-1,3-dicarboxamide</span>;</li> <li class='image_legend_li'>5 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>6 copies of <span class='highlight'>S-1,2-PROPANEDIOL</span>;</li> <li class='image_legend_li'>7 copies of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>D-MALATE</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul> PDB entry 4hma coloured by chain, front view.](https://www.ebi.ac.uk/pdbe/static/entry/4hma_deposited_chain_front_image-200x200.png)
![The deposited structure of PDB entry <span class='highlight'>4hma</span> coloured by chain, side view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>67 kDa matrix metalloproteinase-9</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>CALCIUM ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>N,N'-bis(2-[(biphenyl-4ylsulfonyl)[(2R)-1-hydroxy-3-methyl-1-oxobutan-2-yl]-amino]ethyl)benzene-1,3-dicarboxamide</span>;</li> <li class='image_legend_li'>5 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>6 copies of <span class='highlight'>S-1,2-PROPANEDIOL</span>;</li> <li class='image_legend_li'>7 copies of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>D-MALATE</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul> PDB entry 4hma coloured by chain, side view.](https://www.ebi.ac.uk/pdbe/static/entry/4hma_deposited_chain_side_image-200x200.png)
![The deposited structure of PDB entry <span class='highlight'>4hma</span> coloured by chain, top view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>67 kDa matrix metalloproteinase-9</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>CALCIUM ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>N,N'-bis(2-[(biphenyl-4ylsulfonyl)[(2R)-1-hydroxy-3-methyl-1-oxobutan-2-yl]-amino]ethyl)benzene-1,3-dicarboxamide</span>;</li> <li class='image_legend_li'>5 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>6 copies of <span class='highlight'>S-1,2-PROPANEDIOL</span>;</li> <li class='image_legend_li'>7 copies of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>D-MALATE</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul> PDB entry 4hma coloured by chain, top view.](https://www.ebi.ac.uk/pdbe/static/entry/4hma_deposited_chain_top_image-200x200.png)
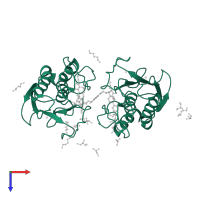
![Homo dimeric assembly 1 of PDB entry <span class='highlight'>4hma</span> coloured by chemically distinct molecules, front view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>67 kDa matrix metalloproteinase-9</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>CALCIUM ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>N,N'-bis(2-[(biphenyl-4ylsulfonyl)[(2R)-1-hydroxy-3-methyl-1-oxobutan-2-yl]-amino]ethyl)benzene-1,3-dicarboxamide</span>;</li> <li class='image_legend_li'>5 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>6 copies of <span class='highlight'>S-1,2-PROPANEDIOL</span>;</li> <li class='image_legend_li'>7 copies of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>D-MALATE</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Homo dimeric assembly 1 of PDB entry 4hma coloured by chemically distinct molecules, front view.](https://www.ebi.ac.uk/pdbe/static/entry/4hma_assembly_1_chemically_distinct_molecules_front_image-200x200.png)
![Homo dimeric assembly 1 of PDB entry <span class='highlight'>4hma</span> coloured by chemically distinct molecules, side view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>67 kDa matrix metalloproteinase-9</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>CALCIUM ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>N,N'-bis(2-[(biphenyl-4ylsulfonyl)[(2R)-1-hydroxy-3-methyl-1-oxobutan-2-yl]-amino]ethyl)benzene-1,3-dicarboxamide</span>;</li> <li class='image_legend_li'>5 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>6 copies of <span class='highlight'>S-1,2-PROPANEDIOL</span>;</li> <li class='image_legend_li'>7 copies of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>D-MALATE</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Homo dimeric assembly 1 of PDB entry 4hma coloured by chemically distinct molecules, side view.](https://www.ebi.ac.uk/pdbe/static/entry/4hma_assembly_1_chemically_distinct_molecules_side_image-200x200.png)
![Homo dimeric assembly 1 of PDB entry <span class='highlight'>4hma</span> coloured by chemically distinct molecules, top view. This structure contains: <ul class='image_legend_ul'><li class='image_legend_li'>2 copies of <span class='highlight'>67 kDa matrix metalloproteinase-9</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>ZINC ION</span>;</li> <li class='image_legend_li'>2 copies of <span class='highlight'>CALCIUM ION</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>N,N'-bis(2-[(biphenyl-4ylsulfonyl)[(2R)-1-hydroxy-3-methyl-1-oxobutan-2-yl]-amino]ethyl)benzene-1,3-dicarboxamide</span>;</li> <li class='image_legend_li'>5 copies of <span class='highlight'>GLYCEROL</span>;</li> <li class='image_legend_li'>6 copies of <span class='highlight'>S-1,2-PROPANEDIOL</span>;</li> <li class='image_legend_li'>7 copies of <span class='highlight'>DI(HYDROXYETHYL)ETHER</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>D-MALATE</span>;</li> <li class='image_legend_li'>1 copy of <span class='highlight'>water</span>.</li></ul>} Homo dimeric assembly 1 of PDB entry 4hma coloured by chemically distinct molecules, top view.](https://www.ebi.ac.uk/pdbe/static/entry/4hma_assembly_1_chemically_distinct_molecules_top_image-200x200.png)