Function and Biology Details
Reaction catalysed:
Exonucleolytic cleavage in the 3'- to 5'-direction to yield nucleoside 5'-phosphates
Biochemical function:
Biological process:
Cellular component:
Structure analysis Details
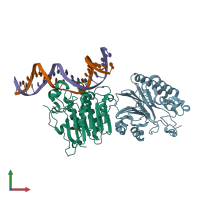
Assemblies composition:
Assembly name:
PDBe Complex ID:
PDB-CPX-119615 (preferred)
Entry contents:
1 distinct polypeptide molecule
2 distinct DNA molecules
2 distinct DNA molecules
Macromolecules (3 distinct):