Function and Biology Details
Reaction catalysed:
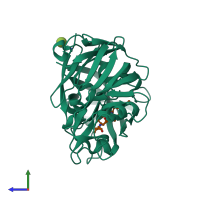
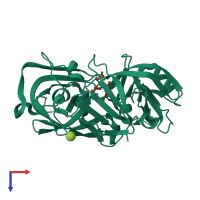
Hydrolysis of proteins with broad specificity similar to that of pepsin A, preferring hydrophobic residues at P1 and P1', but also cleaving 20-Gly-|-Glu-21 in the B chain of insulin. Clots milk, and activates trypsinogen.
Biochemical function:
Biological process:
Cellular component:
Structure analysis Details
Assemblies composition:
Assembly name:
Penicillopepsin-1 and peptide (preferred)
PDBe Complex ID:
PDB-CPX-133573 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):