Function and Biology Details
Reaction catalysed:
Hydrolysis of proteins, with broad specificity for peptide bonds. Native collagen is cleaved about 75% of the length of the molecule from the N-terminus. Low activity on small molecule substrates of both trypsin and chymotrypsin.
Biochemical function:
Biological process:
Cellular component:
Sequence domains:
- Proteinase inhibitor I11, ecotin
- Peptidase S1A, chymotrypsin family
- Serine proteases, trypsin domain
- Proteinase inhibitor I11, ecotin, gammaproteobacteria
- Peptidase S1, PA clan, chymotrypsin-like fold
- Ecotin, C-terminal
- Ecotin superfamily
- Serine proteases, trypsin family, serine active site
2 more domains
Structure domains:
Structure analysis Details
Assembly composition:
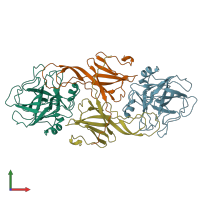
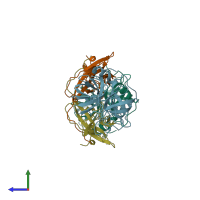
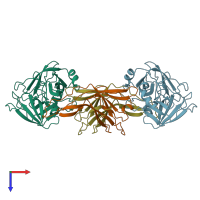
hetero tetramer (preferred)
Assembly name:
Ecotin and Brachyurin (preferred)
PDBe Complex ID:
PDB-CPX-133450 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):
Ligands and Environments
No bound ligands
No modified residues
Experiments and Validation Details
Spacegroup:
P3221