Function and Biology Details
Reactions catalysed:
2-dehydro-3-deoxy-6-phosphate-D-gluconate = pyruvate + D-glyceraldehyde 3-phosphate
(4R)-4-hydroxy-2-oxoglutarate = pyruvate + glyoxylate
Biochemical function:
Biological process:
- not assigned
Cellular component:
Sequence domains:
Structure domain:
Structure analysis Details
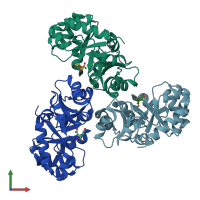
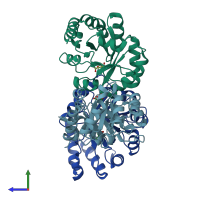
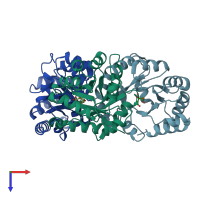
Assembly composition:
homo trimer (preferred)
Assembly name:
KHG/KDPG aldolase (preferred)
PDBe Complex ID:
PDB-CPX-141815 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule: