Function and Biology Details
Reactions catalysed:
RH + [reduced NADPH--hemoprotein reductase] + O(2) = ROH + [oxidized NADPH--hemoprotein reductase] + H(2)O
NADPH + n oxidized hemoprotein = NADP(+) + n reduced hemoprotein
Biochemical function:
Biological process:
- not assigned
Cellular component:
- not assigned
Sequence domains:
Structure domain:
Structure analysis Details
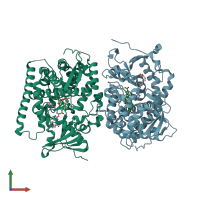
Assembly composition:
monomeric (preferred)
Assembly name:
PDBe Complex ID:
PDB-CPX-147079 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule: