Function and Biology Details
Reaction catalysed:
ATP + L-seryl/L-threonyl/L-tyrosyl-[protein] = ADP + O-phospho-L-seryl/O-phospho-L-threonyl/O-phospho-L-tyrosyl-[protein]
Biochemical function:
- not assigned
Biological process:
- not assigned
Cellular component:
- not assigned
Sequence domains:
Structure domain:
Structure analysis Details
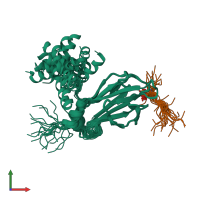
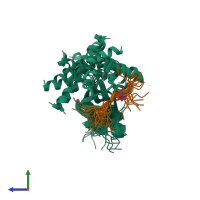
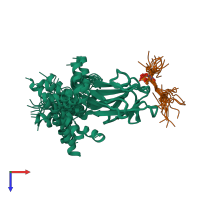
Assembly composition:
hetero dimer (preferred)
Assembly name:
PDBe Complex ID:
PDB-CPX-147050 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):
Ligands and Environments
No bound ligands
1 modified residue:
Experiments and Validation Details
Refinement method:
The complex structure are generated using a total of 2474 restraints. Among them, 3 artificial constraints, 192 TALOS-derived dihedral angle restraints, 78 H-bond restraints, 22 intermolecular distance constrains, and 2179 intra-FHA1 and intra-peptide distance constraints
Expression systems:
- Escherichia coli BL21(DE3)
- Not provided