Function and Biology Details
Reaction catalysed:
Hydrolysis of --Pro-|- and to a lesser extent --Ala-|- in oligopeptides.
Biochemical function:
Biological process:
Cellular component:
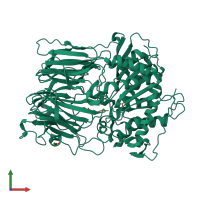
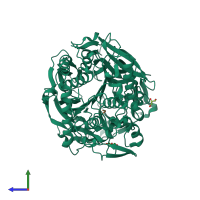
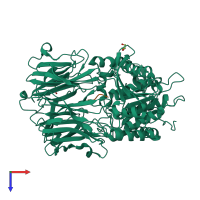
Structure analysis Details
Assembly composition:
hetero dimer (preferred)
Assembly name:
Prolyl endopeptidase and peptide (preferred)
PDBe Complex ID:
PDB-CPX-150046 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):