Function and Biology Details
Reaction catalysed:
Endohydrolysis of (1->4)-beta-D-xylosidic linkages in xylans
Biochemical function:
Biological process:
Cellular component:
- not assigned
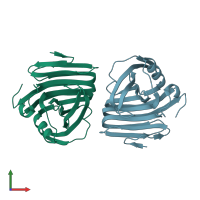
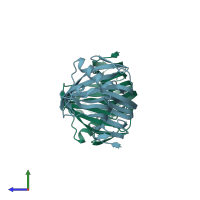
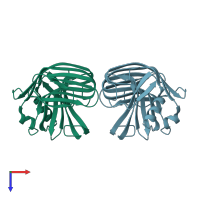
Structure analysis Details
Assembly composition:
monomeric (preferred)
Assembly name:
Endo-1,4-beta-xylanase (preferred)
PDBe Complex ID:
PDB-CPX-181966 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule:
Ligands and Environments
No bound ligands
No modified residues
Experiments and Validation Details
X-ray source:
RIGAKU RU200
Spacegroup:
P212121