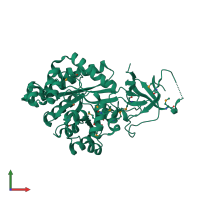
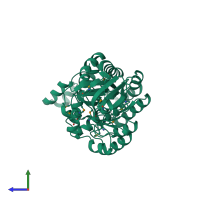
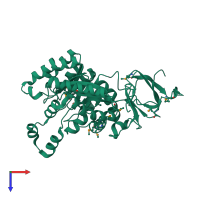
Function and Biology Details
Reaction catalysed:
(S)-3-(5-oxo-4,5-dihydro-3H-imidazol-4-yl)propanoate + H(2)O = N-formimidoyl-L-glutamate + H(+)
Biochemical function:
Biological process:
Cellular component:
Structure analysis Details
Assemblies composition:
Assembly name:
Imidazolonepropionase (preferred)
PDBe Complex ID:
PDB-CPX-106095 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule: