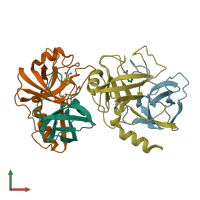
Function and Biology Details
Reaction catalysed:
Preferential cleavage of Arg-|- bonds in small molecule substrates. Highly selective action to release kallidin (lysyl-bradykinin) from kininogen involves hydrolysis of Met-|- or Leu-|-. The rat enzyme is unusual in liberating bradykinin directly from autologous kininogens by cleavage at two Arg-|- bonds (5).
Biochemical function:
Biological process:
Cellular component:
- not assigned
Sequence domains:
Structure domain:
Structure analysis Details
Assemblies composition:
Assembly name:
Glandular kallikrein (preferred)
PDBe Complex ID:
PDB-CPX-133312 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):