X-ray diffraction
2.2Å resolution
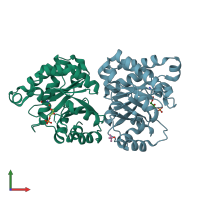
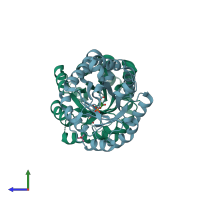
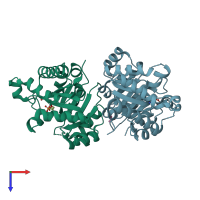
Structure-based enzyme engineering efforts with an inactive monomeric TIM variant: the importance of a single point mutation for generating an active site with suitable binding properties
Released:
DOI:
10.2210/pdb2vem/pdb
Source organism:
Trypanosoma brucei brucei
Primary publication: