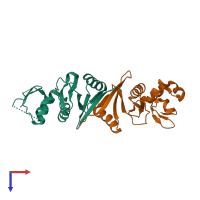
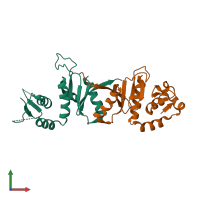Function and Biology Details
Reaction catalysed:
PretRNA = a 3'-half-tRNA molecule with a 5'-OH end + a 5'-half-tRNA molecule with a 2',3'-cyclic phosphate end + an intron with a 2',3'-cyclic phosphate and a 5'-hydroxyl terminus
Biochemical function:
Biological process:
Cellular component:
- not assigned
Sequence domains:
- tRNA intron endonuclease, catalytic domain-like
- tRNA intron endonuclease, catalytic domain-like superfamily
- tRNA-splicing endonuclease
- tRNA endonuclease-like domain superfamily
- tRNA intron endonuclease, N-terminal
- tRNA-splicing endonuclease, archaeal short subfamily
- tRNA intron endonuclease, N-terminal domain superfamily
Structure analysis Details
Assemblies composition:
Assembly name:
PDBe Complex ID:
PDB-CPX-181081 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):
Ligands and Environments
No bound ligands
No modified residues
Experiments and Validation Details
X-ray source:
APS BEAMLINE 22-ID
Spacegroup:
I41
Expression system: Escherichia coli