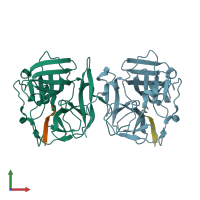Function and Biology Details
Reactions catalysed:
Nucleoside triphosphate + RNA(n) = diphosphate + RNA(n+1)
Hydrolyzes glutaminyl bonds, and activity is further restricted by preferences for the amino acids in P6 - P1' that vary with the species of potyvirus, e.g. Glu-Xaa-Xaa-Tyr-Xaa-Gln-|-(Ser or Gly) for the enzyme from tobacco etch virus. The natural substrate is the viral polyprotein, but other proteins and oligopeptides containing the appropriate consensus sequence are also cleaved.
Hydrolyzes a Gly-|-Gly bond at its own C-terminus, commonly in the sequence -Tyr-Xaa-Val-Gly-|-Gly, in the processing of the potyviral polyprotein.
Biochemical function:
Biological process:
Cellular component:
- not assigned
Structure analysis Details
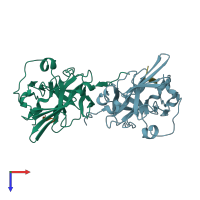
Assemblies composition:
Assembly name:
Capsid protein (preferred)
PDBe Complex ID:
PDB-CPX-140760 (preferred)
Entry contents:
2 distinct polypeptide molecules
Macromolecules (2 distinct):