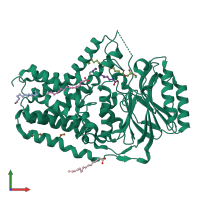
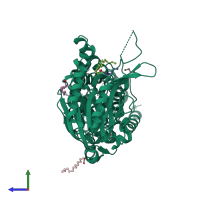
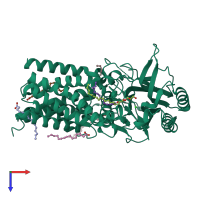
Function and Biology Details
Reaction catalysed:
A phosphoglycerolipid + an [apolipoprotein]-S-1,2-diacyl-sn-glyceryl-L-cysteine = a 1-lyso-phosphoglycerolipid + a [lipoprotein]-N-acyl-S-1,2-diacyl-sn-glyceryl-L-cysteine
Biochemical function:
Biological process:
Cellular component:
Structure analysis Details
Assembly composition:
monomeric (preferred)
Assembly name:
Apolipoprotein N-acyltransferase (preferred)
PDBe Complex ID:
PDB-CPX-150121 (preferred)
Entry contents:
1 distinct polypeptide molecule
Macromolecule: